Everything you need to know about CE marking
Are you launching a new product in Europe? Then you should know about CE marking. For many types of products, CE marking is a requirement for the product to be sold legally in the EU and EEA – and an important seal of quality.
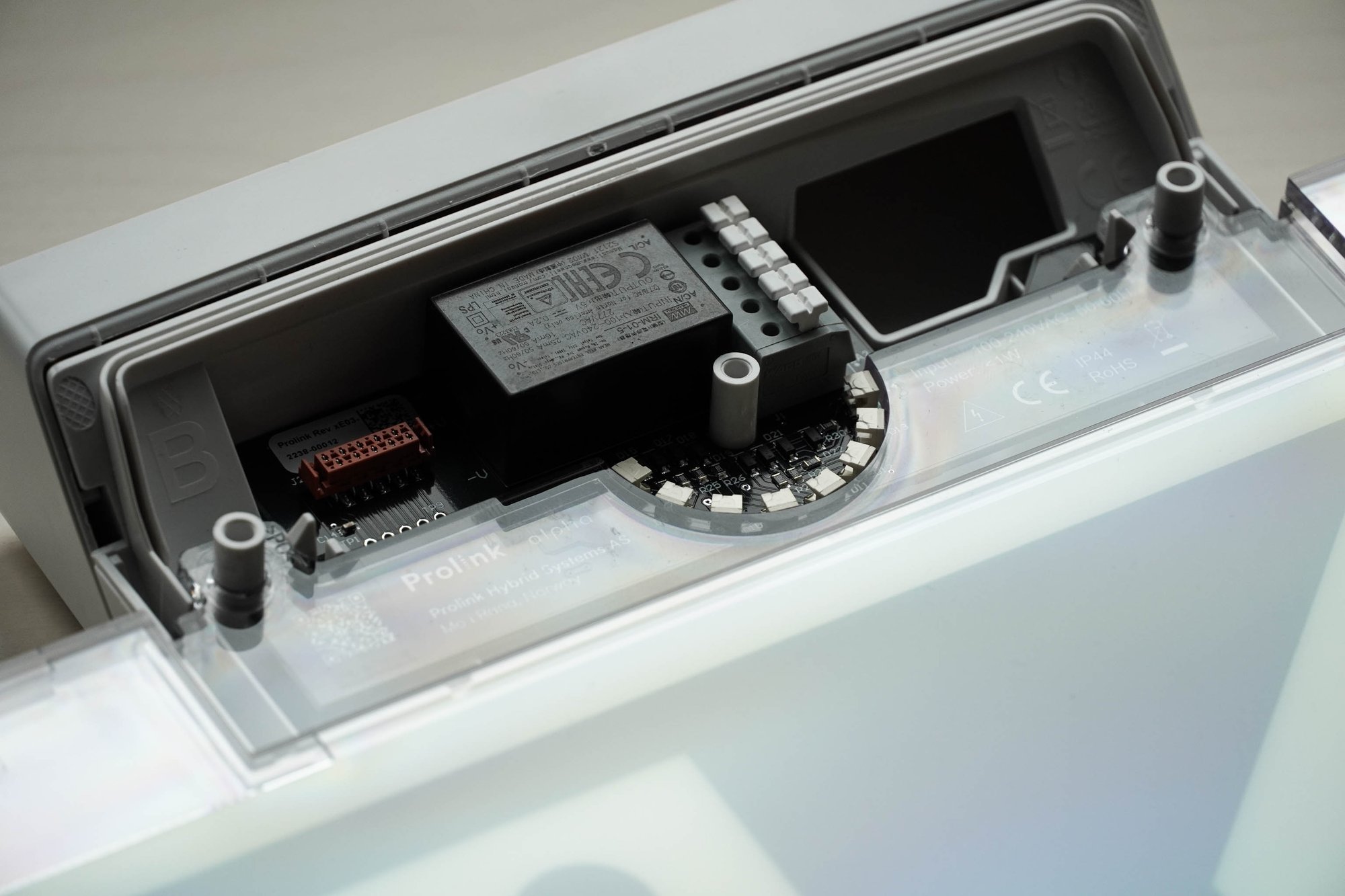
What is CE marking – and why does it concern you?
For the manufacturer, CE marking means responsibility – and access to the market. For the customer, it means security.
The CE mark is proof that your product meets health, safety and environmental requirements. It shows that you have done the work: assessed the risks, tested the product, documented the processes and ensured that it is safe to use.
If there is a CE marking requirement, it is important to know that the process leading to CE marking is not the same for everyone. The requirements that apply depend on the type of product you are developing. A health app, an electrical component and a syringe pump all follow different rules. Some must follow the Low Voltage Directive, others the MDR Regulation, and some must go through the Machinery or Construction Products Directive.
Some products can be CE marked with a self-declaration. Others require a technical inspection body to assess and approve the documentation. However, the documentation must be in place.
At Inventas, we combine regulatory understanding with technical insight. This means we can help you make smart choices – both for the product and for the documentation.
We help you with:
- find out what requirements apply to your product
- structure the documentation correctly from the start
- avoid bottlenecks and misunderstandings along the way
- develop a realistic plan for testing and approval
- ensure compliance with relevant standards and regulations
We are happy to work closely with you all the way – from early concept development to finished documentation and CE marking. Our consultants, designers, engineers and regulatory specialists collaborate across disciplines to ensure you get a product that both works and meets the requirements.
You get a multidisciplinary team that knows both regulations and development processes – and that does everything to ensure that you get a good and approved product onto the market.
Three typical races – and what you need to think about
CE marking is about safety – both for you as the developer and for those who will use the product. Here are three typical product categories we often help with.
Electronics
Electronic products often have to meet the requirements of three key directives:
- Low Voltage Directive (LVD) – requirements for electrical safety during normal use.
- EMC Directive – requirements that the product does not interfere with or is interfered with by other electronics.
- RoHS Directive – environmental requirements for the content of harmful substances.
To meet the requirements, you must document the product's properties, perform relevant tests and create technical documentation – often based on harmonized standards.
At Inventas, we build electronic solutions from idea to finished product. We work with everything from IoT and sensors to FPGAs and ASICs, and have a strong professional environment within testing, control and regulation. This means that we also know the requirements – and how to best meet them.
We help you with:
- choosing which standards and tests apply
- development of printed circuit boards and embedded systems
- test strategy and documentation flow
- assessment of manufacturability and conformity
Software and AI
If you develop software that is to be used for medical purposes (e.g. diagnostics, health monitoring or treatment), then this is considered a medical device, and then there is a requirement for CE marking. Then you must follow the Medical Device Regulation (MDR) in order to CE mark the software. When the software includes artificial intelligence, the process becomes more complex.
You must document:
- what the software does, how it is built and what data it uses
- how the algorithms work and are validated
- that data security, privacy and traceability are ensured
For AI-based products, new requirements will be introduced through the AI Act, which introduces risk categories and stricter requirements for transparency, monitoring and testing. At Inventas, we follow developments closely and help you set up your documentation so that it meets both current and future requirements.
Medical equipment
If you are developing medical devices, you must comply with both the CE marking requirements and the MDR – Medical Device Regulation. The MDR is a regulation that applies throughout the EU/EEA and sets its own strict requirements for medical devices. It regulates how the product should be developed, documented and followed up – and is therefore a central part of the CE marking for this type of product. The MDR places higher demands on traceability, safety and documentation, and involves a much more comprehensive assessment of the product's risk, function and performance.
Among other things, you must – depending on the product's properties and area of application:
- classify the product in the correct risk class (I to III), depending on how much risk the product may pose and how it will be used
- establish and follow a quality system in line with ISO 13485 (a standard for safe and structured development of medical devices)
- prepare a technical file with specifications, clinical evaluation, risk assessment and test documentation
- involve a technical inspection body for assessment (applies to all classes except low risk)
Inventas has been ISO 13485 certified since 2022. This means that we have the systems, procedures and experience needed to support you every step of the way. You get a development partner who knows both the requirements and what it actually takes to meet them – in practice. We also help you find out if your product is actually a medical device – for the many grey area products.
The technical file – the path to CE marking of medical devices
CE marking is a requirement for the product to be sold legally.
We know the requirements can seem extensive – but it doesn’t have to be complicated. Let’s chat about where you are in the process and how we can help you further. Fill out the form to have a no-obligation chat with us about how we can find the solution together.
Are you going to CE mark a new product?
Per-Anders Elvertrø
per-anders.elvertro@inventas.no
90 69 83 34
Tore Eide
tore.eide@inventas.no
90 56 91 33

